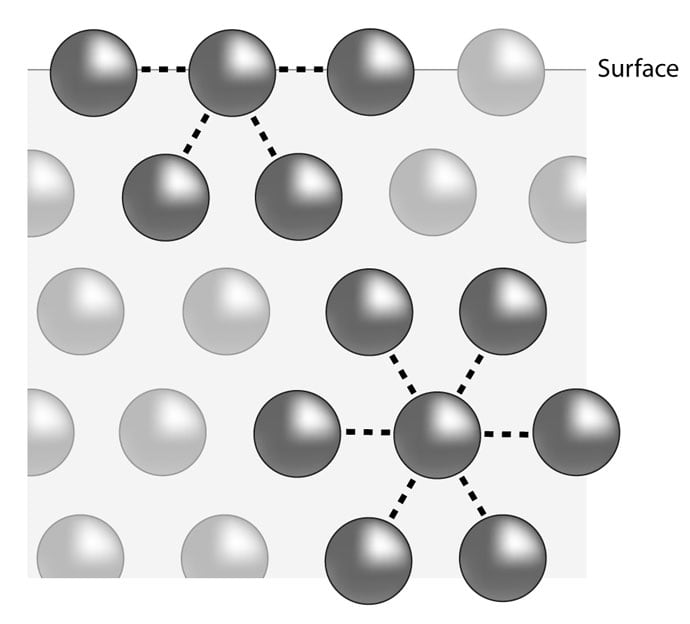
Surface Tension Of Water Means . learn about surface tension, the property of a liquid surface that acts like a stretched elastic membrane. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on water. learn how surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. surface tension is the tendency of fluid surfaces to shrink into the minimum area possible due to intermolecular. surface tension is the amount of energy required to increase the surface area of a liquid, caused by intermolecular forces. Learn how it affects water properties, examples, and applications with. surface tension is the result of cohesive forces between liquid molecules at the surface. learn what surface tension is, how it is caused by intermolecular forces, and how it affects.
from www.artworkarchive.com
Learn how it affects water properties, examples, and applications with. surface tension is the amount of energy required to increase the surface area of a liquid, caused by intermolecular forces. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on water. learn about surface tension, the property of a liquid surface that acts like a stretched elastic membrane. surface tension is the tendency of fluid surfaces to shrink into the minimum area possible due to intermolecular. learn what surface tension is, how it is caused by intermolecular forces, and how it affects. surface tension is the result of cohesive forces between liquid molecules at the surface. learn how surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces.
Surface Tension of Water 2 from the collection of Guild of Natural
Surface Tension Of Water Means Learn how it affects water properties, examples, and applications with. learn what surface tension is, how it is caused by intermolecular forces, and how it affects. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on water. Learn how it affects water properties, examples, and applications with. learn how surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. surface tension is the tendency of fluid surfaces to shrink into the minimum area possible due to intermolecular. learn about surface tension, the property of a liquid surface that acts like a stretched elastic membrane. surface tension is the result of cohesive forces between liquid molecules at the surface. surface tension is the amount of energy required to increase the surface area of a liquid, caused by intermolecular forces.
From studyfullirene.z21.web.core.windows.net
Surface Tension Of Water Worksheet Surface Tension Of Water Means surface tension is the result of cohesive forces between liquid molecules at the surface. learn about surface tension, the property of a liquid surface that acts like a stretched elastic membrane. surface tension is the tendency of fluid surfaces to shrink into the minimum area possible due to intermolecular. surface tension is the amount of energy. Surface Tension Of Water Means.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Surface Tension Of Water Means surface tension is the tendency of fluid surfaces to shrink into the minimum area possible due to intermolecular. learn how surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. surface tension is the result of cohesive forces between liquid molecules at the surface. learn what surface tension. Surface Tension Of Water Means.
From thehomeschoolscientist.com
Surface Tension In Water Explanation and Experiment The Homeschool Surface Tension Of Water Means surface tension is the tendency of fluid surfaces to shrink into the minimum area possible due to intermolecular. surface tension is the result of cohesive forces between liquid molecules at the surface. Learn how it affects water properties, examples, and applications with. learn how surface tension is the energy required to increase the surface area of a. Surface Tension Of Water Means.
From www.youtube.com
What is surface tension of water called? YouTube Surface Tension Of Water Means surface tension is the tendency of fluid surfaces to shrink into the minimum area possible due to intermolecular. surface tension is the result of cohesive forces between liquid molecules at the surface. Learn how it affects water properties, examples, and applications with. learn what surface tension is, how it is caused by intermolecular forces, and how it. Surface Tension Of Water Means.
From ar.inspiredpencil.com
Surface Tension Water Surface Tension Of Water Means surface tension is the result of cohesive forces between liquid molecules at the surface. learn what surface tension is, how it is caused by intermolecular forces, and how it affects. learn how surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. surface tension is the tendency of. Surface Tension Of Water Means.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids Surface Tension Of Water Means learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on water. Learn how it affects water properties, examples, and applications with. learn how surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. learn what surface tension. Surface Tension Of Water Means.
From ar.inspiredpencil.com
Surface Tension Water Surface Tension Of Water Means learn how surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. surface tension is the tendency of fluid surfaces to shrink into the minimum area possible due to intermolecular. learn what surface tension is, how it is caused by intermolecular forces, and how it affects. surface tension. Surface Tension Of Water Means.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse Surface Tension Of Water Means Learn how it affects water properties, examples, and applications with. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on water. learn what surface tension is, how it is caused by intermolecular forces, and how it affects. learn how surface tension is the energy required. Surface Tension Of Water Means.
From www.biolinscientific.com
Surface tension of water Why is it so high? Surface Tension Of Water Means surface tension is the amount of energy required to increase the surface area of a liquid, caused by intermolecular forces. Learn how it affects water properties, examples, and applications with. surface tension is the result of cohesive forces between liquid molecules at the surface. learn about surface tension, the property of a liquid surface that acts like. Surface Tension Of Water Means.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download Surface Tension Of Water Means surface tension is the amount of energy required to increase the surface area of a liquid, caused by intermolecular forces. Learn how it affects water properties, examples, and applications with. surface tension is the tendency of fluid surfaces to shrink into the minimum area possible due to intermolecular. learn how surface tension is the energy required to. Surface Tension Of Water Means.
From www.youtube.com
Surface Tension Experiment Water and Our World The Good and the Surface Tension Of Water Means learn what surface tension is, how it is caused by intermolecular forces, and how it affects. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on water. surface tension is the tendency of fluid surfaces to shrink into the minimum area possible due to intermolecular.. Surface Tension Of Water Means.
From www.youtube.com
Surface Tension of Water Explained YouTube Surface Tension Of Water Means surface tension is the amount of energy required to increase the surface area of a liquid, caused by intermolecular forces. learn about surface tension, the property of a liquid surface that acts like a stretched elastic membrane. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to. Surface Tension Of Water Means.
From www.usgs.gov
Surface Tension and Water U.S. Geological Survey Surface Tension Of Water Means learn how surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. surface tension is the tendency of fluid surfaces to shrink into the minimum area possible due to intermolecular. Learn how it affects water properties, examples, and applications with. learn about surface tension, the property of a liquid. Surface Tension Of Water Means.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? Surface Tension Of Water Means learn about surface tension, the property of a liquid surface that acts like a stretched elastic membrane. surface tension is the amount of energy required to increase the surface area of a liquid, caused by intermolecular forces. Learn how it affects water properties, examples, and applications with. learn how water molecules cohere to form a strong surface. Surface Tension Of Water Means.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记1.5.3 Hydrogen Bonding翰林国际教育 Surface Tension Of Water Means Learn how it affects water properties, examples, and applications with. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on water. surface tension is the result of cohesive forces between liquid molecules at the surface. surface tension is the tendency of fluid surfaces to shrink. Surface Tension Of Water Means.
From circuitenginemeseta101.z22.web.core.windows.net
Diagram Of Surface Tension Surface Tension Of Water Means learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on water. Learn how it affects water properties, examples, and applications with. surface tension is the amount of energy required to increase the surface area of a liquid, caused by intermolecular forces. learn about surface tension,. Surface Tension Of Water Means.
From www.moleaer.com
How nanobubbles improve water infiltration and by reducing Surface Tension Of Water Means Learn how it affects water properties, examples, and applications with. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on water. learn how surface tension is the energy required to increase the surface area of a liquid due to intermolecular forces. learn about surface tension,. Surface Tension Of Water Means.
From mavink.com
Water Surface Tension Chart Surface Tension Of Water Means learn about surface tension, the property of a liquid surface that acts like a stretched elastic membrane. surface tension is the amount of energy required to increase the surface area of a liquid, caused by intermolecular forces. Learn how it affects water properties, examples, and applications with. surface tension is the tendency of fluid surfaces to shrink. Surface Tension Of Water Means.